Here are the solutions to the sample exam questions on waves & radiation.
 mrmackenzie
mrmackenzie
National 5 exam questions – waves and radiation
As promised, here are the Waves and Radiation exam questions from previous Standard Grade Credit and Intermediate 2 papers. A pdf of solutions will follow.
higher – unit 3 practice NAB solutions
I’ve attached the solutions to the practice NAB. Come and see me if you need any help.
higher – unit 3 practice NAB
Here is a practice NAB for unit 3. Please try the whole test before looking at the answers!
Rutherford’s model of the atom
We’ve been looking at how Ernest Rutherford showed that Thomson’s plum pudding (think of Christmas pudding or fruit cake) model of the atom was incorrect by firing alpha particles at a piece of thin gold foil. Although most alpha particles passed straight through, some were scattered at large angles, or even came back.
This evidence allowed Rutherford to develop the idea of a nuclear atom, where the mass is concentrated in a small volume at the centre with a positive charge. The overall charge on the atom remains neutral due to electrons with a negative charge orbiting the nucleus. Most of Rutherford’s atomic model was empty space!
Here is a screenshot of the animation I used in class, the red spheres represent the alpha particles fired at the gold foil. Click on the picture to play the animatino sequence in full, watching what happens to the alpha particles after they reach the gold target.
Here’s a youtube video that explains Rutherford scattering and looks at how we can manipulate the nucleus of an atom to turn it from one element into another, a process called transmutation.
Professor Brian Cox goes back to Rutherford’s old laboratory in Manchester in this short video.
higher – unit 1 practice NAB answers
Here are the answers to the pratice NAB for unit 1. How did you get on?
higher – practice unit 1 NAB
Here is the practice NAB for unit 1. I will post the answers on Sunday morning. I suggest you work thourgh the unit 1 revision materials before attempting this assessment.
Don’t cheat! Do the NAB before looking at the answers.
higher – lasers
The first laser was demonstrated in 1960 by Theodore Maiman and his research group at Hughes Aircraft Corporation† in California. Here is a good background article on the first laser, its inventor and the role that Einstein played in developing the theory of stimulated emission.
The principle of laser operation is outlined in this description of Maiman’s laser, which used a rod of polished ruby inside a spiral flashtube.
My favourite James Bond film, Goldfinger, has a scene where Sean Connery (the best 007 imho) is strapped to a table under a huge red laser. It should have been a saw but the invention of the laser, just 4 years earlier, was a gift for the writers. This scene helped the film win the best effects Oscar in 1965 and, more importantly, gave us the ultimate Bond quote:
Bond: Do you expect me to talk?
Goldfinger: No, Mr. Bond, I expect you to die.
Everyone should watch the laser scene.
Bonus points if you can tell me about the bad physics in that clip…
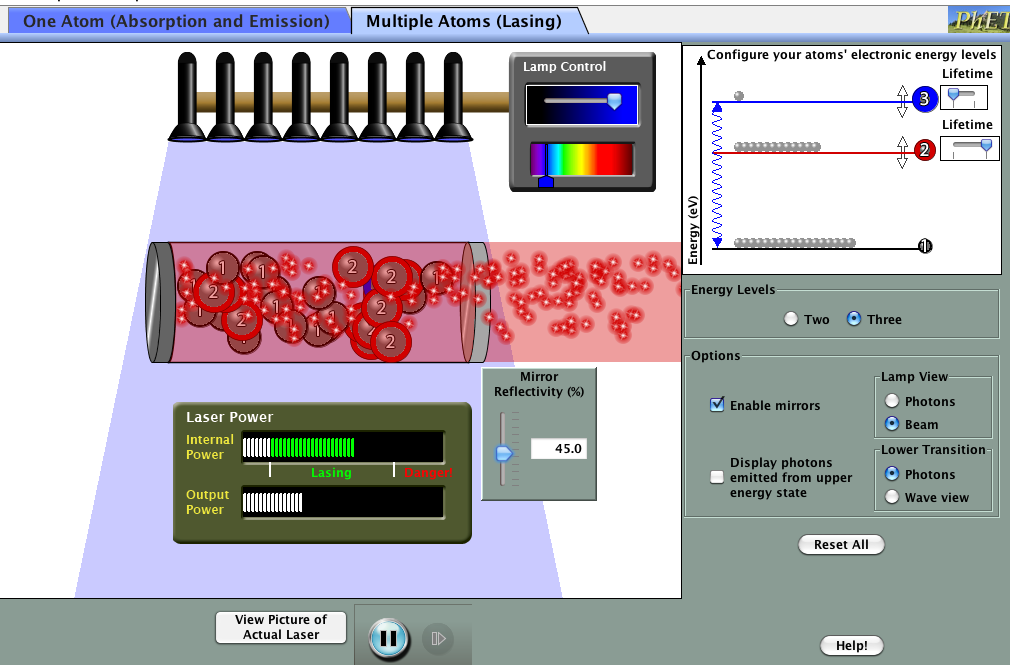
You can try running a laser for yourself. Click on the picture below to load a simulator. You’ll need Java on your computer to run the simulation.
Try changing lamp (pump) irradiance and mirror reflectivity on the single atom version before moving on to the multiple atom tab.
There are some pdf notes on lasers attached to the end of this post.
† Disclaimer: I used to work for Hughes before I trained as a physics teacher – the Glenrothes branch, not California 🙁
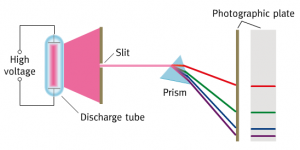
higher – line spectra
We’ve looked at line spectra with spectroscopes and a spectrometer recently. Now we’re considering how these different colours of light are produced.
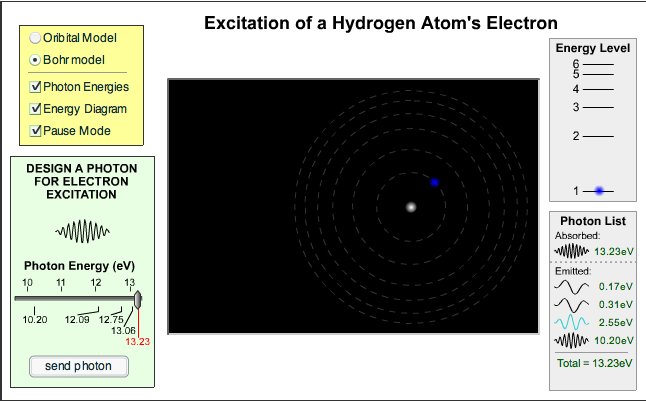
Here is a website that lets you choose the energy of a photon and see whether or not it causes a change in the energy of an electron inside the hydrogen atom. Try it – the labels under the slider for photon energy match the electron transitions.
You can read more about line spectra and their origin here.
The visible line spectrum of the Hydrogen atom is explained in this short animation. Click on the image below to start the clip.
While you’re watching the clip, try to explain why the video does not include ANY of the possible transistions back down to the ground state.
I’ve attached a pdf file with further notes on line spectra and the absorption/emission of photons. Click on the link below to download your copy.
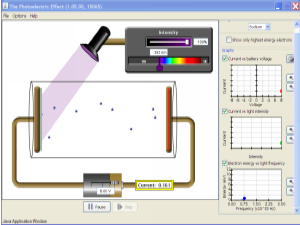
Higher – photoelectric effect
We looked at the photoelectric effect earlier this week. This video has a similar demonstration to the gold leaf electroscope experiment I showed you in class and includes an explanation of the process.
Click on the picture below to download the simulation we used to investigate the effect of irradiance on frequency on photocurrent. You’ll be prompted to install Java if you don’t have it already.
Once the animation is running, you can;
- change the metal under investigation (we used zinc in class)
- vary the wavelength of the incident light
- vary the irradiance of the incident light.
Notice that below the theshold frequency you can’t get any photoelectrons, even if you set the light to its brightest setting.
Compare your results to the graphs provided in your notes.
I have attached some notes & questions on the photoelectric effect. Click on the link below to download a copy.