Here is the link you will need for the task in class tomorrow.
Higher
uranium-235 and nuclear fission
Uranium has several isotopes, all of which are naturally radioactive. The two most abundant isotopes are
- U-238 (92 protons, 146 neutrons) 99.28% of uranium is this isotope
- U-235 (92 protons, 143 neutrons) 0.71% of uranium is this isotope
Although less than 1% of all the uranium we can dig out of the ground is the U-235 isoptope, this is the one that gets most attention. Atoms of U-235 have a special property, they can absorb low energy neutrons and then split up into two smaller atoms. This process is called fission. The animation below is a representation of the nucleus of a U-235 atom absorbing a passing (blue) neutron and splitting into two fission fragments as a result.
Animated gif showing fission of U-235 by Stephan-Xp
The total mass of the original U-235 atom and the single neutron is greater than the combined mass of the two fission fragments and the three new neutrons. The “missing mass” is converted into energy. For each U-235 atom that undergoes fission, a large quantity of energy is released. We can calculate how much energy is released using Einstein’s famous equation
Notice how the fission process shown above produces 3 new neutrons in addition to the fission fragments. These neutrons can go on to be absorbed by other U-235 atoms, causing further fission.
If the fission process is controlled, by limiting the number of available neutrons, then it can be used in a nuclear power station. Without that control, fission occurs rapidly and all of the energy is released in a very short period, resulting in a nuclear explosion.
Only the U-235 isotope is fissile (able to undergo fission) and mined uranium must be processed to remove the U-238 isotope. This filtering process is called enrichment and Professor Poliakoff from periodicvideos.com has a video that explains the process but thankfully not in enough detail for you to build your own nuclear bomb!
Rutherford’s model of the atom
We’ve been looking at how Ernest Rutherford showed that Thomson’s plum pudding (think of Christmas pudding or fruit cake) model of the atom was incorrect by firing alpha particles at a piece of thin gold foil. Although most alpha particles passed straight through, some were scattered at large angles, or even came back.
This evidence allowed Rutherford to develop the idea of a nuclear atom, where the mass is concentrated in a small volume at the centre with a positive charge. The overall charge on the atom remains neutral due to electrons with a negative charge orbiting the nucleus. Most of Rutherford’s atomic model was empty space!
Here is a screenshot of the animation I used in class, the red spheres represent the alpha particles fired at the gold foil. You can get the full animation by clicking on the download link at the end of this post. Watch what happens to them after they reach the gold.
Here’s a youtube video that explains Rutherford scattering and looks at how we can manipulate the nucleus of an atom to turn it from one element into another, a process called transmutation.
Professor Brian Cox goes back to Rutherford’s old laboratory in Manchester in this short video.
In these video clips, you will hear the name of one of Rutherford’s students, Hans Geiger, mentioned. Hans Geiger went on to invent the radiation detector that is named after him. In the following clip, taken from a chemistry class at a Canadian university, we see the counter in use by Geiger’s great grandson.
fun with hot gas
Impress your friends at parties with this physics trick.
Now you’ve watched it, go back and listen carefully to the explanation in step 3. Notice how each step is explained in sequence. This is the “cause and effect” type of description we discussed last week.
Also notice how the egg is always pushed, it is never “sucked”. Suck is bad physics!
Higher – improving descriptions and explanations
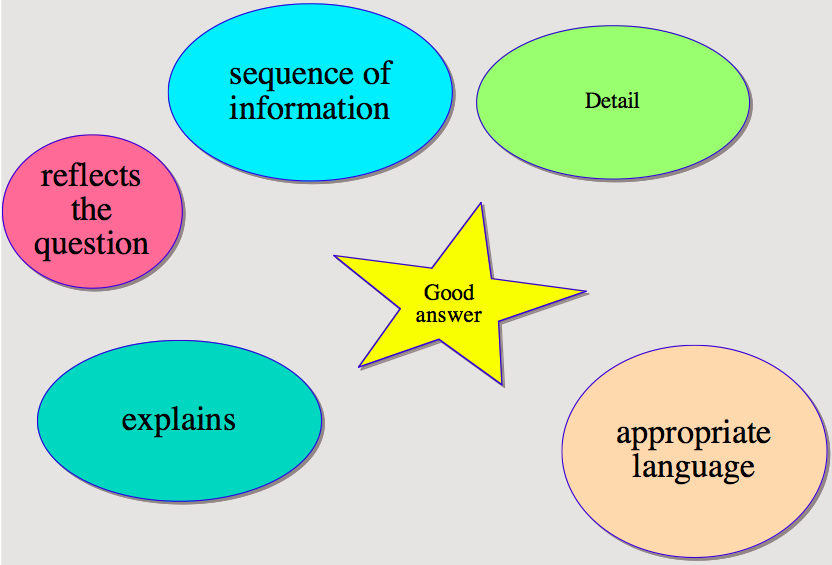
Mind mapping can be a useful technique for displaying information. We spent some time last week looking at ways to raise the quality of your written responses to describe & explain questions.
The first task generated themes for a mind map and we went on to use the map to help structure your descriptions in a peer assessment task, where you provided feedback to improve each other’s answers. Here is the mind map you produced.
I have attached a copy of the group tasks we used as a pdf file.
Higher homework- forces & momentum
Here is your homework exercise on forces and momentum. Please hand your hw jotter in no later than Monday 16th November.
Newton III in action
On Friday, we examined the importance of Newton’s 3rd law of motion. In our discussions, different explanations for the motion of jets and rockets were proposed and considered. The front runners were;
- at launch, the ground pushes back against a rocket
- during flight, air pushes back against a plane
Unfortunately, the lack of ground and air (or any other gas) meant that neither of these models were able to explain the propulsion of an object in space. It was at this point we remembered Newton’s 3rd law of motion (or here with non-rocket examples) from Standard Grade.
You’ve got to be careful with Newton’s 3rd law of motion, it’s easy to get confused. Bonus question: What’s wrong with this explanation?
I found a photograph that provides a stunning visualisation of Newton’s 3rd law in action during the launch of a DeltaIV rocket. You can read the details of setting up for this photo here.
image courtesy of Ben Cooper, Launchphotography.com
The photo was taken at very short range (about 30m) from the launch site and clearly shows hot gases being forced out of the exhaust at high speed. When a rocket forces out gas, the expelled gas pushes back on the exhaust with an equal force. Since the exhaust is part of the rocket’s structure, the entire rocket is propelled in the opposite direction to the gas.
It is this pushing back on the exhaust that provides thrust for a rocket. It doesn’t matter if the rocket is on the launch pad, in mid air or outer space. As long as it can push gas out of the exhaust, it will be able to propel itself forwards using Newton’s 3rd law of motion.
We don’t normally get a clear view of the hot gases being forced out of a rocket in launch photographs. A lot of the smoke seen in images like the one shown below is actually steam.
NASA/courtesy of nasaimages.org
There are two main sources of steam during launch. The most obvious is the burning of fuels but NASA also soaks launch platforms with water just before and after launch so that the massive sound waves don’t damage the vehicle being launched. There is a wikipedia article on the use of water during space shuttle launches.
LO3 advice
Those of you working on your LO3 report this weekend might like to see what another physics teacher, Mr Hood, has to say about this aspect of your course work. Read his blog post here. He’s also kindly provided more detailed help in the form of an audio podcast. Click the link below to listen.
momentum
momentum introduction from mr mackenzie on Vimeo.
We’ve just started to look at momentum in class. There are 3 basic categories of interaction we will look at;
- inelastic collisions
- elastic collisions
- explosive collisions