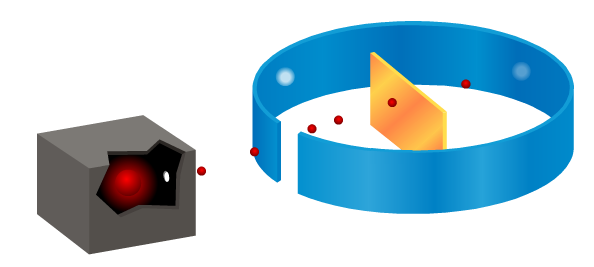
We’ve been looking at how Ernest Rutherford showed that Thomson’s plum pudding (think of Christmas pudding or fruit cake) model of the atom was incorrect by firing alpha particles at a piece of thin gold foil. Although most alpha particles passed straight through, some were scattered at large angles, or even came back.
This evidence allowed Rutherford to develop the idea of a nuclear atom, where the mass is concentrated in a small volume at the centre with a positive charge. The overall charge on the atom remains neutral due to electrons with a negative charge orbiting the nucleus. Most of Rutherford’s atomic model was empty space!
Here is a screenshot of the animation I used in class, the red spheres represent the alpha particles fired at the gold foil. You can get the full animation by clicking on the download link at the end of this post. Watch what happens to them after they reach the gold.
Here’s a youtube video that explains Rutherford scattering and looks at how we can manipulate the nucleus of an atom to turn it from one element into another, a process called transmutation.
Professor Brian Cox goes back to Rutherford’s old laboratory in Manchester in this short video.
In these video clips, you will hear the name of one of Rutherford’s students, Hans Geiger, mentioned. Hans Geiger went on to invent the radiation detector that is named after him. In the following clip, taken from a chemistry class at a Canadian university, we see the counter in use by Geiger’s great grandson.