Uranium has several isotopes, all of which are naturally radioactive. The two most abundant isotopes are
- U-238 (92 protons, 146 neutrons) 99.28% of uranium is this isotope
- U-235 (92 protons, 143 neutrons) 0.71% of uranium is this isotope
Although less than 1% of all the uranium we can dig out of the ground is the U-235 isoptope, this is the one that gets most attention. Atoms of U-235 have a special property, they can absorb low energy neutrons and then split up into two smaller atoms. This process is called fission. The animation below is a representation of the nucleus of a U-235 atom absorbing a passing (blue) neutron and splitting into two fission fragments as a result.
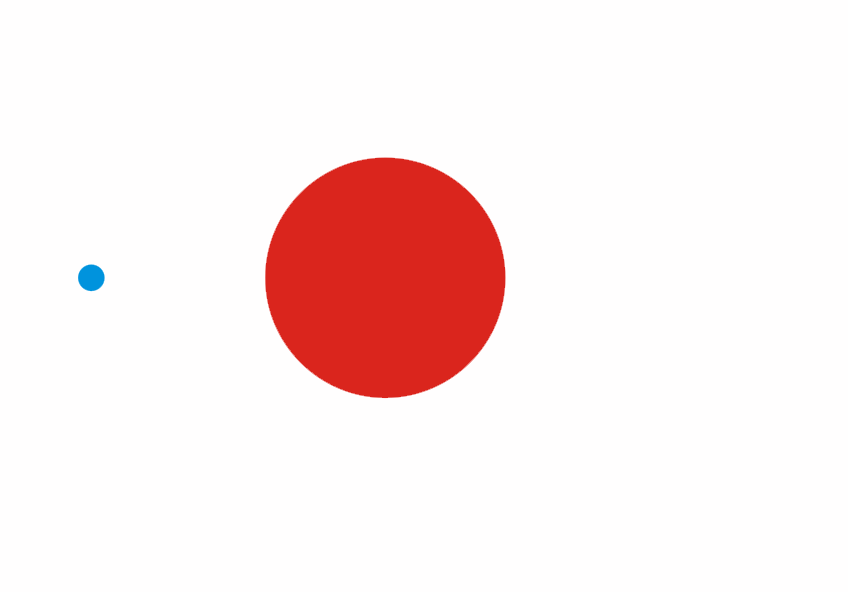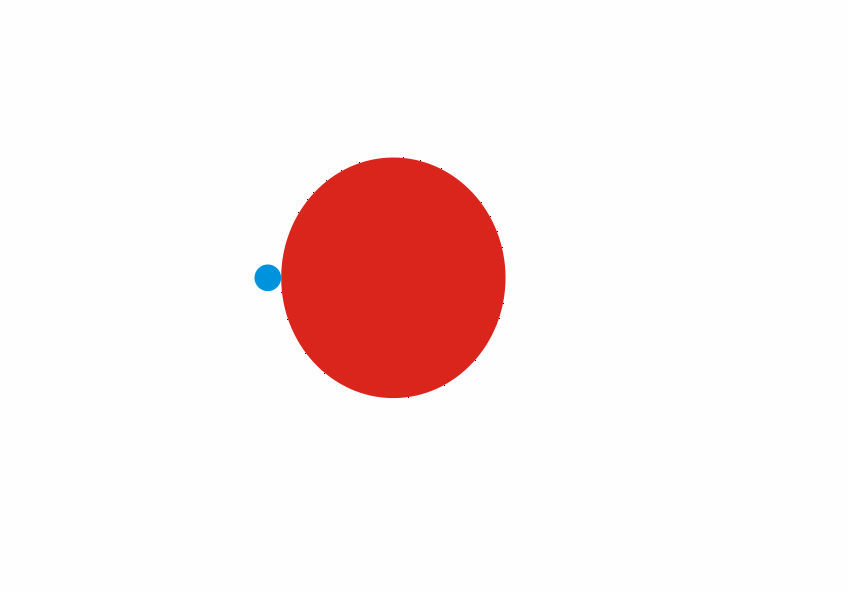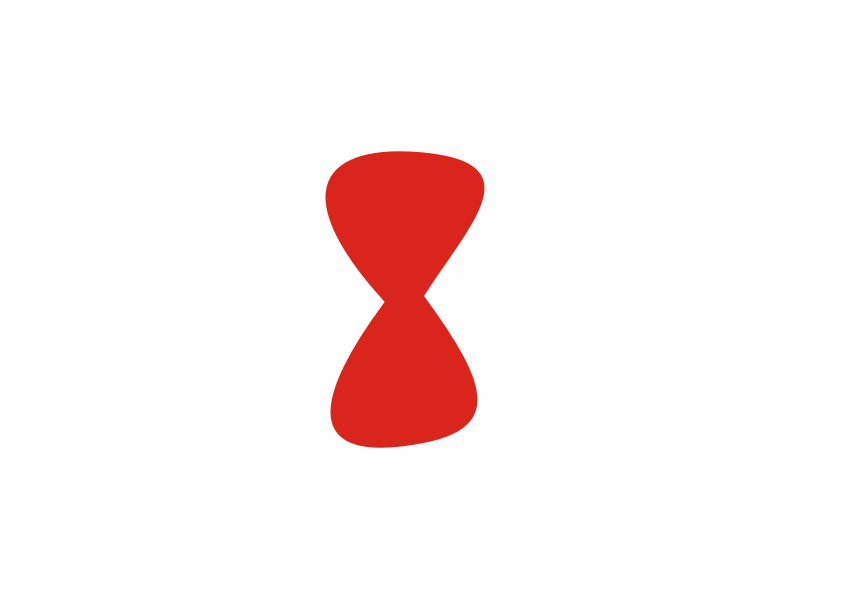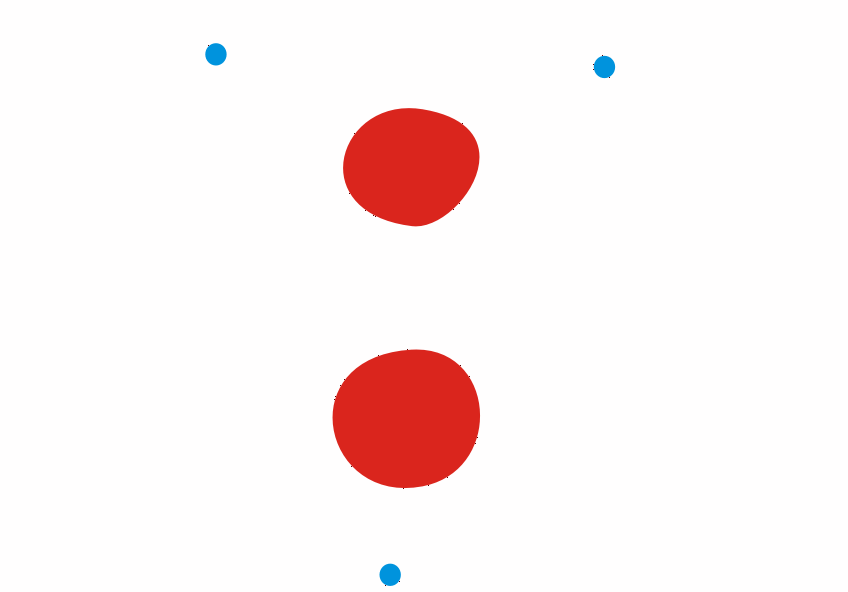
Animated gif showing fission of U-235 by Stephan-Xp
The total mass of the original U-235 atom and the single neutron is greater than the combined mass of the two fission fragments and the three new neutrons. The “missing mass” is converted into energy. For each U-235 atom that undergoes fission, a large quantity of energy is released. We can calculate how much energy is released using Einstein’s famous equation
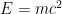
Notice how the fission process shown above produces 3 new neutrons in addition to the fission fragments. These neutrons can go on to be absorbed by other U-235 atoms, causing further fission.
If the fission process is controlled, by limiting the number of available neutrons, then it can be used in a nuclear power station. Without that control, fission occurs rapidly and all of the energy is released in a very short period, resulting in a nuclear explosion.
Only the U-235 isotope is fissile (able to undergo fission) and mined uranium must be processed to remove the U-238 isotope. This filtering process is called enrichment and Professor Poliakoff from periodicvideos.com has a video that explains the process but thankfully not in enough detail for you to build your own nuclear bomb!