mrmackenzie
mrmackenzie
radioactivity task
Here is the link you will need for the task in class tomorrow.
uranium-235 and nuclear fission
Uranium has several isotopes, all of which are naturally radioactive. The two most abundant isotopes are
- U-238 (92 protons, 146 neutrons) 99.28% of uranium is this isotope
- U-235 (92 protons, 143 neutrons) 0.71% of uranium is this isotope
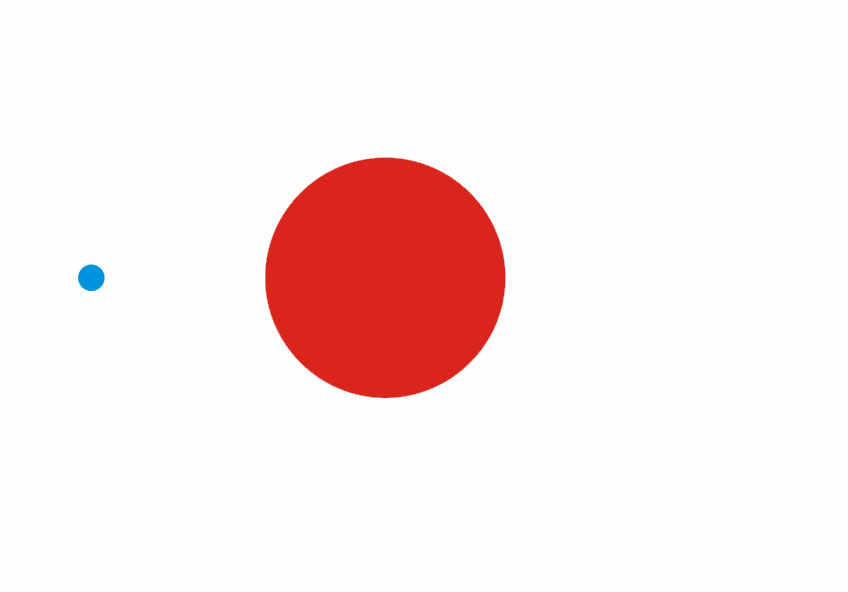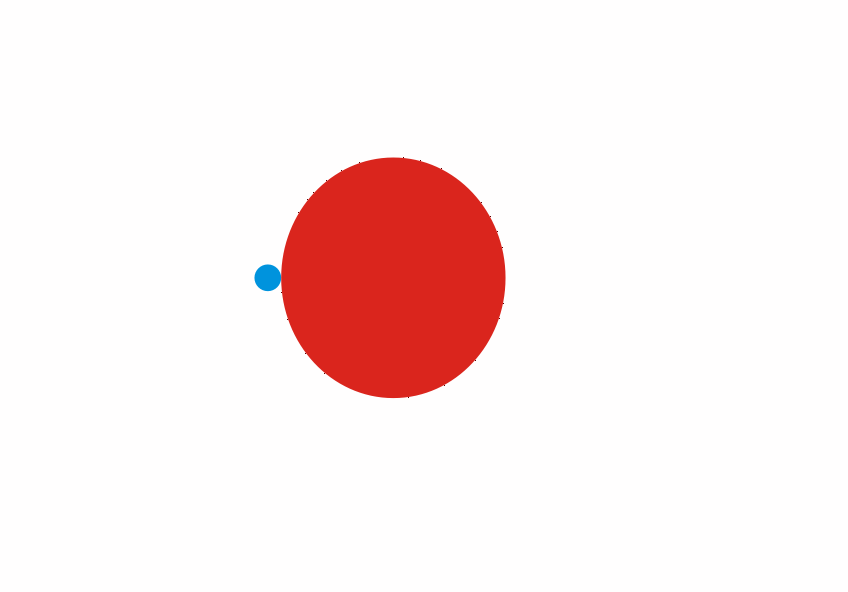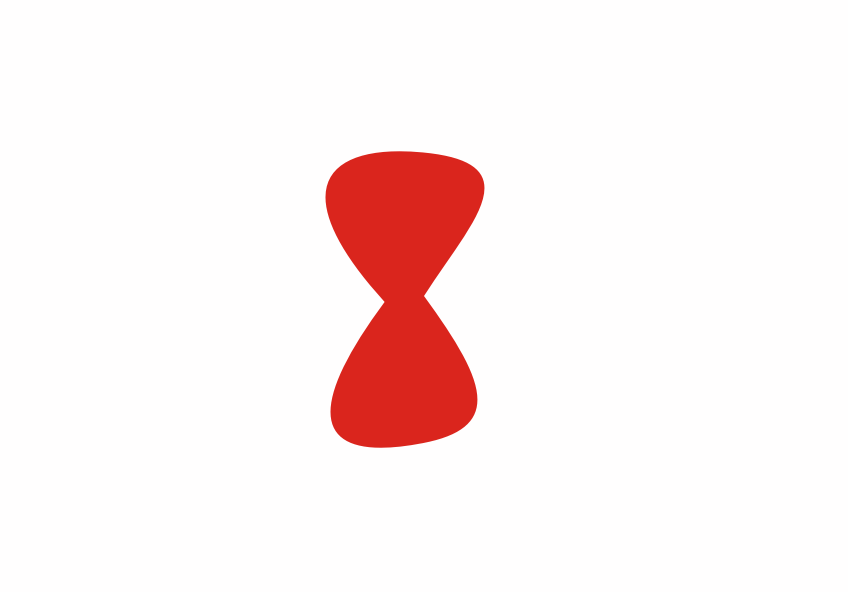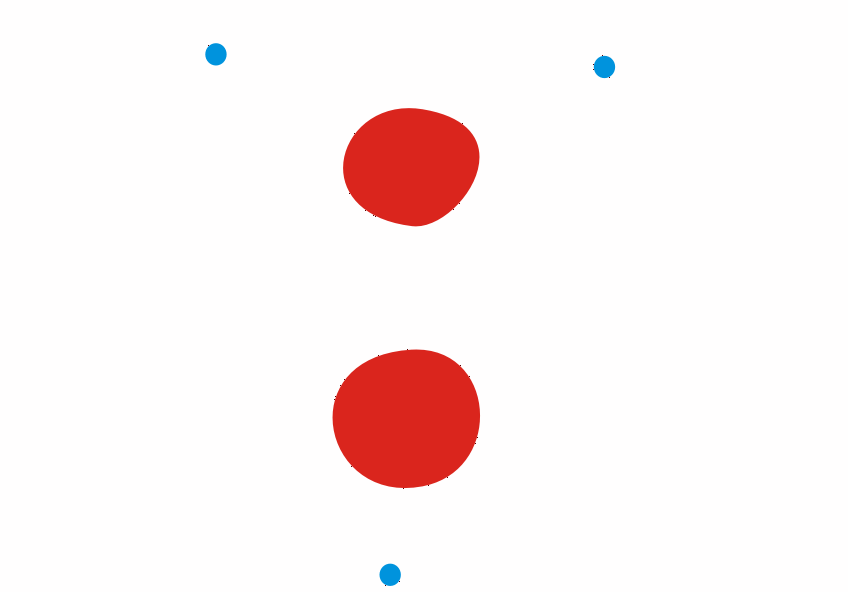
Although less than 1% of all the uranium we can dig out of the ground is the U-235 isoptope, this is the one that gets most attention. Atoms of U-235 have a special property, they can absorb low energy neutrons and then split up into two smaller atoms. This process is called fission. The animation below is a representation of the nucleus of a U-235 atom absorbing a passing (blue) neutron and splitting into two fission fragments as a result.
Animated gif showing fission of U-235 by Stephan-Xp
The total mass of the original U-235 atom and the single neutron is greater than the combined mass of the two fission fragments and the three new neutrons. The “missing mass” is converted into energy. For each U-235 atom that undergoes fission, a large quantity of energy is released. We can calculate how much energy is released using Einstein’s famous equation
Notice how the fission process shown above produces 3 new neutrons in addition to the fission fragments. These neutrons can go on to be absorbed by other U-235 atoms, causing further fission.
If the fission process is controlled, by limiting the number of available neutrons, then it can be used in a nuclear power station. Without that control, fission occurs rapidly and all of the energy is released in a very short period, resulting in a nuclear explosion.
Only the U-235 isotope is fissile (able to undergo fission) and mined uranium must be processed to remove the U-238 isotope. This filtering process is called enrichment and Professor Poliakoff from periodicvideos.com has a video that explains the process but thankfully not in enough detail for you to build your own nuclear bomb!
Rutherford’s model of the atom
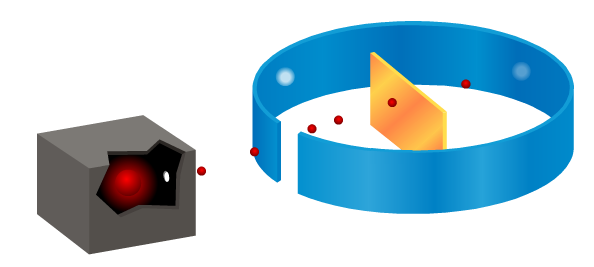
We’ve been looking at how Ernest Rutherford showed that Thomson’s plum pudding (think of Christmas pudding or fruit cake) model of the atom was incorrect by firing alpha particles at a piece of thin gold foil. Although most alpha particles passed straight through, some were scattered at large angles, or even came back.
This evidence allowed Rutherford to develop the idea of a nuclear atom, where the mass is concentrated in a small volume at the centre with a positive charge. The overall charge on the atom remains neutral due to electrons with a negative charge orbiting the nucleus. Most of Rutherford’s atomic model was empty space!
Here is a screenshot of the animation I used in class, the red spheres represent the alpha particles fired at the gold foil. You can get the full animation by clicking on the download link at the end of this post. Watch what happens to them after they reach the gold.
Here’s a youtube video that explains Rutherford scattering and looks at how we can manipulate the nucleus of an atom to turn it from one element into another, a process called transmutation.
Professor Brian Cox goes back to Rutherford’s old laboratory in Manchester in this short video.
In these video clips, you will hear the name of one of Rutherford’s students, Hans Geiger, mentioned. Hans Geiger went on to invent the radiation detector that is named after him. In the following clip, taken from a chemistry class at a Canadian university, we see the counter in use by Geiger’s great grandson.
fun with hot gas
Impress your friends at parties with this physics trick.
Now you’ve watched it, go back and listen carefully to the explanation in step 3. Notice how each step is explained in sequence. This is the “cause and effect” type of description we discussed last week.
Also notice how the egg is always pushed, it is never “sucked”. Suck is bad physics!
Millikan’s oil drop experiment
On Monday, we discussed Millikan’s famous experiment to determine the charge on an electron. Some of the slides I used during the lesson were taken from a site created by Joe Rowing, who teaches physics in Brighton. You can download the full powerpoint file and handout sheets here.

We performed a small experiment to show how Millikan was able to calculate the charge due to a single electron. Balloons filled with different numbers of beads replaced the charged oil drops and allowed us to simulate Millikan’s method using an electronic balance. The mass of a single bead at 0.22g – congratulations to those of you who were able to reach this value!
If you look at the slides on the site I linked to above, you will find a description of the experiment I based this on. Mr Rowing used sweets instead of beads.
Higher – improving descriptions and explanations
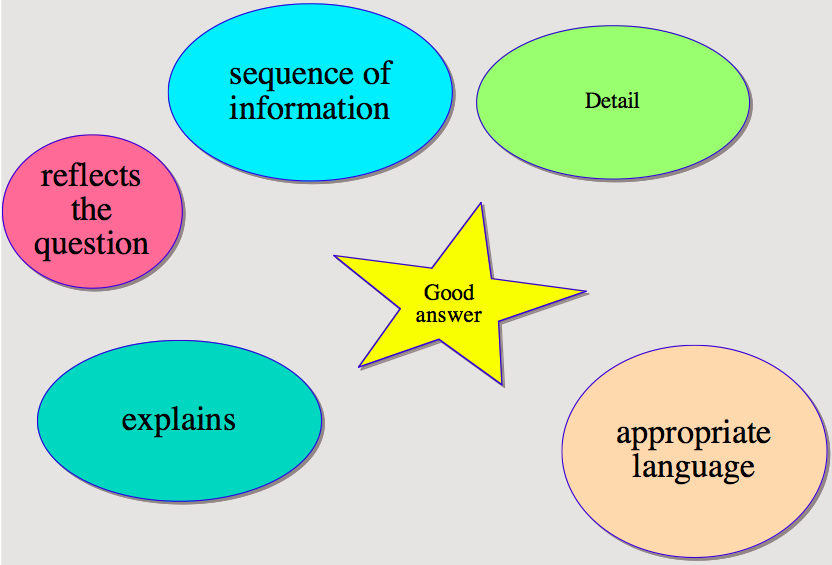
Mind mapping can be a useful technique for displaying information. We spent some time last week looking at ways to raise the quality of your written responses to describe & explain questions.
The first task generated themes for a mind map and we went on to use the map to help structure your descriptions in a peer assessment task, where you provided feedback to improve each other’s answers. Here is the mind map you produced.
I have attached a copy of the group tasks we used as a pdf file.