The first laser was demonstrated in 1960 by Theodore Maiman and his research group at Hughes† in California. Here is a good background article on the first laser, its inventor and the role that Einstein played in developing the theory of stimulated emission.
The principle of laser operation is outlined in this description of Maiman’s laser, which used a rod of polished ruby inside a spiral flashtube.
My favourite James Bond film, Goldfinger, has a scene where Sean Connery (the best 007 imho) is strapped to a table under a huge red laser. It should have been a saw but the invention of the laser, just 4 years earlier, was a gift for the writers. This scene helped the film win the best effects Oscar in 1965 and, more importantly, gave us the ultimate Bond quote:
Bond: Do you expect me to talk?
Goldfinger: No, Mr. Bond, I expect you to die.
Everyone should watch the laser scene.
Bonus points if you can tell me about the bad physics in that clip…
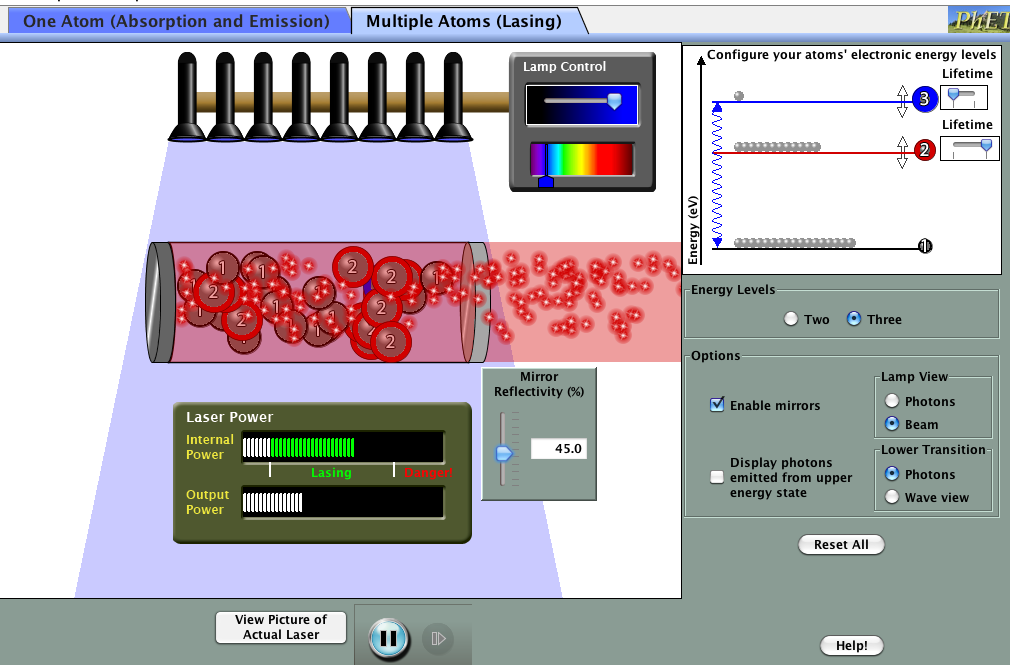
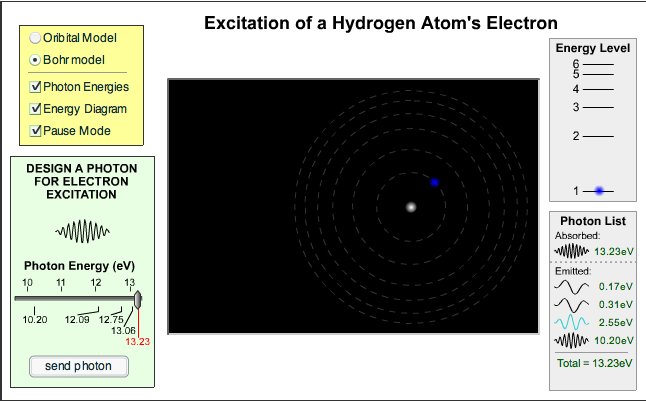
You can try running a laser for yourself. Click on the picture below to load a simulator. You’ll need Java on your computer to run the simulation.
Try changing lamp (pump) irradiance and mirror reflectivity on the single atom version before moving on to the multiple atom tab.
There are some pdf notes on lasers attached to the end of this post.
† Disclaimer: I used to work for Hughes before I trained as a physics teacher – the Glenrothes branch, not California 🙁