Answers to Properties of Matter Homework
Return to Higher Physics past paper index page.
Density
1. r = 7.7g/cm3 or 7,700kg/m3
2. r = 9,142.86kg/m3
3. V = 0.02378m3
4. m =1.36x10-3kg (NB:100ml = 100cm3 = 100x10-6m3)
5. V = 1.0017x10-3m3 = 1001.7ml
6. 83.6mm
7. Lithium would be 42.36 times longer than osmium.
8. The particle spacing in solids and liquids of the same
substance is about the same. However, in the gaseous state
the particle spacing is 10x greater. This means that for a given
mass of a substance the solid and liquid would occupy roughly the
same volume and have an equal density, but the same mass of gas
would occupy a volume 1000x greater (V=10x10x10) and have a density
1000x less. (r = m/V)
9. E (1993 PI Q11)
10. 1.103kg/m3 (1993 PI Q32)
11. 11.9
12. Low density polystyrene displaces higher density water from the ship.
Reducing the overall density of the ship to less than that of the water
will make the ship float.
13. Weight of 1m3 of lead = mg =107800N
To make the lead float in mercury this means that the
mercury must produce an upthrust of 107800N.
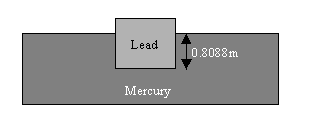 The upward pressure on the lower surface of the lead,
produced by the mercury liquid, must produce an upward
force that will balance the weight.
Fliquid = PliquidA = (rgh)A
Fliquid = weight
(rgh)A = mg
=>h = m/rA
=>h = (11,000kg/m3)/[(13600kg/m3)x(1m2)]
=>h = 0.8088m
Pressure
14. P = 2,450Pa
15. F = 6237N
16. E (1992 PI Q10)
17. E (1995 PI Q9)
18. B (1999 PI Q8)
19. C (1996 PI Q10)
20. m = 50kg
21. F =176,000N
22. A = 1x10-2m2 (using g=10N/kg)
23.a. A sharp blade has a small surface area.
This creates a large pressure when a force
is applied to the knife because: P = F/A.
b. Skis increase the contact area with the snow.
The pressure produced by the weight of the person
is therefore reduced (P = F/A).
24. P = 18849.56N
25. Fres = 15x104N (to the left) (1993 PI Q32)
Pressure in a Fluid
26. E (1998 PI Q8)
27. B (1992 PI Q12)
Buoyancy/Upthrust
28. A (1997 PI Q10)
29. D (1999 PI Q6)
30. E (1992 PI Q6)
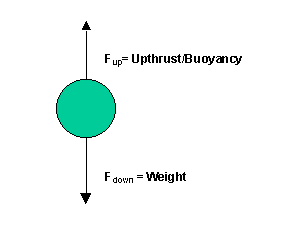
31. All forces on the balloon are balanced [N1].
The weight of the balloon is equal and opposite
to the upthrust force.
32a. (1997 PI Q32a)
The upward pressure on the lower surface of the lead,
produced by the mercury liquid, must produce an upward
force that will balance the weight.
Fliquid = PliquidA = (rgh)A
Fliquid = weight
(rgh)A = mg
=>h = m/rA
=>h = (11,000kg/m3)/[(13600kg/m3)x(1m2)]
=>h = 0.8088m
Pressure
14. P = 2,450Pa
15. F = 6237N
16. E (1992 PI Q10)
17. E (1995 PI Q9)
18. B (1999 PI Q8)
19. C (1996 PI Q10)
20. m = 50kg
21. F =176,000N
22. A = 1x10-2m2 (using g=10N/kg)
23.a. A sharp blade has a small surface area.
This creates a large pressure when a force
is applied to the knife because: P = F/A.
b. Skis increase the contact area with the snow.
The pressure produced by the weight of the person
is therefore reduced (P = F/A).
24. P = 18849.56N
25. Fres = 15x104N (to the left) (1993 PI Q32)
Pressure in a Fluid
26. E (1998 PI Q8)
27. B (1992 PI Q12)
Buoyancy/Upthrust
28. A (1997 PI Q10)
29. D (1999 PI Q6)
30. E (1992 PI Q6)
31. All forces on the balloon are balanced [N1].
The weight of the balloon is equal and opposite
to the upthrust force.
32a. (1997 PI Q32a)
 b. 0N (1997 PI Q32b)
33a.i. (1996 PI Q32ai)
b. 0N (1997 PI Q32b)
33a.i. (1996 PI Q32ai)
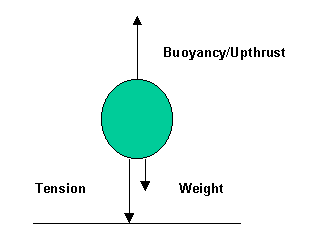
 a.ii. Fbuoyancy = 1250N
b. Fbuoyancy = 1250N (buoyancy force not dependent on depth)
34a. w = 49000N (1995 PII Q4a)
b.i. Buoyancy = 20000N (1995 PII Q4bi/ii)
b.ii.
c. No effect (1995 PII Q4c)
35a.i. (1994 PII Q2)
a.ii. Fbuoyancy = 1250N
b. Fbuoyancy = 1250N (buoyancy force not dependent on depth)
34a. w = 49000N (1995 PII Q4a)
b.i. Buoyancy = 20000N (1995 PII Q4bi/ii)
b.ii.
c. No effect (1995 PII Q4c)
35a.i. (1994 PII Q2)
 a.ii. Buoyancy = 5650N
a.iii.T = 750N
b. Trope 1 = 413.8N
Trope 2 = 413.8N
c. Cold air is more dense than hot air and for a given
volume has a greater mass than hot air. Exchanging hot
air for cold air increases the mass and weight of the
balloon. The weight of the balloon will now be greater
than the upthrust. This unbalanced force would result in
a downward acceleration.
36a. (1999 PII Q4a)
a.ii. Buoyancy = 5650N
a.iii.T = 750N
b. Trope 1 = 413.8N
Trope 2 = 413.8N
c. Cold air is more dense than hot air and for a given
volume has a greater mass than hot air. Exchanging hot
air for cold air increases the mass and weight of the
balloon. The weight of the balloon will now be greater
than the upthrust. This unbalanced force would result in
a downward acceleration.
36a. (1999 PII Q4a)
 b.i. (1999 PII Q4bi)
b.ii. mass = 14790Kg
b.iii. (1999 PII Q4biii)
Temperature Scales
37. 0oC = 273K
20oC = 293K
100oC = 373K
-50oC = 223K
-173oC = 100K
38. 300K = 27oC
400K = 127oC
650K = 377oC
173K = -100oC
4K = -269oC
39. A (1993 PI Q9)
40a. 77K (1994 PI Q33a)
b. (1994 PI Q33b)
Pressure and Temperature
41a. The pressure of a gas is directly proportional to its
kelvin temperature.
[P1/T1 = P2/T2]
b. Gas pressure is a result of collisions between gas
particles and the walls of their container. As the
temperature of the gas increases the kinetic energy
of the particles increases. The faster moving particles
collide more frequently, and forcefully, with the walls
of their container. The increase in average force per unit
area of container wall is the reason for the pressure
increase.
42.
b.i. (1999 PII Q4bi)
b.ii. mass = 14790Kg
b.iii. (1999 PII Q4biii)
Temperature Scales
37. 0oC = 273K
20oC = 293K
100oC = 373K
-50oC = 223K
-173oC = 100K
38. 300K = 27oC
400K = 127oC
650K = 377oC
173K = -100oC
4K = -269oC
39. A (1993 PI Q9)
40a. 77K (1994 PI Q33a)
b. (1994 PI Q33b)
Pressure and Temperature
41a. The pressure of a gas is directly proportional to its
kelvin temperature.
[P1/T1 = P2/T2]
b. Gas pressure is a result of collisions between gas
particles and the walls of their container. As the
temperature of the gas increases the kinetic energy
of the particles increases. The faster moving particles
collide more frequently, and forcefully, with the walls
of their container. The increase in average force per unit
area of container wall is the reason for the pressure
increase.
42.
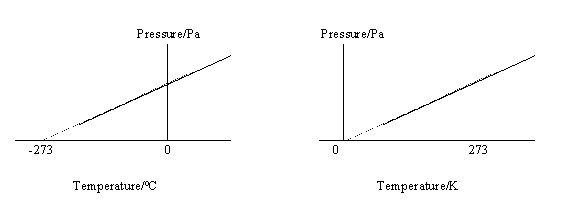 43. -273oC is called absolute zero. It is the coldest
possible temperature.
44. P = 120kPa
45. T = 750K (477oC)
46. C (1998 PI Q9)
47. E (1994 PI Q10)
48. D (1999 PI Q10)
49a.i. (1992 PII Q4ai)
43. -273oC is called absolute zero. It is the coldest
possible temperature.
44. P = 120kPa
45. T = 750K (477oC)
46. C (1998 PI Q9)
47. E (1994 PI Q10)
48. D (1999 PI Q10)
49a.i. (1992 PII Q4ai)
| Pressure/kPa |
89 |
96 |
103 |
110 |
117 |
| Temperature/oC |
-20 |
0 |
20 |
40 |
60 |
| Temperature/K |
253 |
273 |
293 |
313 |
333 |
a.ii. Pressure/Temperature(K) = Constant (1992 PII Q4aii)
a.iii. (1992 PII Q4aiii)
b. T = 68.3K (1992 PII Q4b)
Volume and Temperature
50. T = 546K(273oC)
51a. 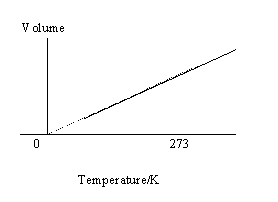 b. V = 0.14cm3
c. Plot a graph of volume against kelvin
temperature for the known temperatures of
boiling water and melting ice.
Record the volume for the freezing mixture
and use the the graph to estimate the kelvin
temperature of the mixture.
Or
Use the relationship:
V1/T1 = V2/T2
And solve for the unknown temperature(T2) by substituting
in known values of V1,T1,V2 and solving for T2.
d. The relationship: V1/T1 = V2/T2, assumes the gas is
at constant pressure, equal to atmospheric pressure.
A change in atmospheric pressure would change the gas pressure.
A graph, or constant(P/T), would therefore be required for each
pressure of the gas.
Pressure and Volume
52. E (1992 PI Q11)
53. A (1995 PI Q10)
54. When a gas is compressed into a smaller volume
the particles collide more frequently with the
walls of their container. A geater average
force is therefore exerted by the gas on a
smaller area. This is equivalent to an increase
in pressure.
55. V = 1500(litres)
56. V = 56ml
57a. V = 1800(litres)
b. 900 litres available at 200kPa
time = available volume/volume per minute
t = 900/25
t = 36(minutes)
58. P = 4x105Pa
59. V2 = 3.84m3 (1995 PI Q33)
60a. (1996 PII Q4a)
b. V = 0.14cm3
c. Plot a graph of volume against kelvin
temperature for the known temperatures of
boiling water and melting ice.
Record the volume for the freezing mixture
and use the the graph to estimate the kelvin
temperature of the mixture.
Or
Use the relationship:
V1/T1 = V2/T2
And solve for the unknown temperature(T2) by substituting
in known values of V1,T1,V2 and solving for T2.
d. The relationship: V1/T1 = V2/T2, assumes the gas is
at constant pressure, equal to atmospheric pressure.
A change in atmospheric pressure would change the gas pressure.
A graph, or constant(P/T), would therefore be required for each
pressure of the gas.
Pressure and Volume
52. E (1992 PI Q11)
53. A (1995 PI Q10)
54. When a gas is compressed into a smaller volume
the particles collide more frequently with the
walls of their container. A geater average
force is therefore exerted by the gas on a
smaller area. This is equivalent to an increase
in pressure.
55. V = 1500(litres)
56. V = 56ml
57a. V = 1800(litres)
b. 900 litres available at 200kPa
time = available volume/volume per minute
t = 900/25
t = 36(minutes)
58. P = 4x105Pa
59. V2 = 3.84m3 (1995 PI Q33)
60a. (1996 PII Q4a)
| Pressure/kPa | 100 | 150 | 200 | 250 |
| Volume/cm3 | 14.7 | 9.9 | 7.4 | 5.9 |
| Pressure x Volume/kPacm3 | 1470 | 1485 | 1480 | 1475 |
b. P = 295kPa (using average value of the constant PV)(1996 PII Q4b)
c. (1996 PII Q4c)
d. (1996 PII Q4d)
General Questions
61. D (1999 PI Q9)
62. A (1997 PI Q11)
63a. m = 3.87x10-3kg (1998 PII Q4a)
b.i. F = 2100N (1998 PII Q4bi)
b.ii. P2 = 1.5x105Pa (1998 PII Q4bii)
64a. V2 = 1600cm3 (1993 PII Q4a)
b. The pressure increases. (1993 PII Q4b)
The volume remains constant.
The temperature increases.
c. T2 = 750K (1993 PII Q4c)
Return to Higher Physics past paper index page.
 The upward pressure on the lower surface of the lead,
produced by the mercury liquid, must produce an upward
force that will balance the weight.
Fliquid = PliquidA = (rgh)A
Fliquid = weight
(rgh)A = mg
=>h = m/rA
=>h = (11,000kg/m3)/[(13600kg/m3)x(1m2)]
=>h = 0.8088m
Pressure
14. P = 2,450Pa
15. F = 6237N
16. E (1992 PI Q10)
17. E (1995 PI Q9)
18. B (1999 PI Q8)
19. C (1996 PI Q10)
20. m = 50kg
21. F =176,000N
22. A = 1x10-2m2 (using g=10N/kg)
23.a. A sharp blade has a small surface area.
This creates a large pressure when a force
is applied to the knife because: P = F/A.
b. Skis increase the contact area with the snow.
The pressure produced by the weight of the person
is therefore reduced (P = F/A).
24. P = 18849.56N
25. Fres = 15x104N (to the left) (1993 PI Q32)
Pressure in a Fluid
26. E (1998 PI Q8)
27. B (1992 PI Q12)
Buoyancy/Upthrust
28. A (1997 PI Q10)
29. D (1999 PI Q6)
30. E (1992 PI Q6)
31. All forces on the balloon are balanced [N1].
The weight of the balloon is equal and opposite
to the upthrust force.
32a. (1997 PI Q32a)
The upward pressure on the lower surface of the lead,
produced by the mercury liquid, must produce an upward
force that will balance the weight.
Fliquid = PliquidA = (rgh)A
Fliquid = weight
(rgh)A = mg
=>h = m/rA
=>h = (11,000kg/m3)/[(13600kg/m3)x(1m2)]
=>h = 0.8088m
Pressure
14. P = 2,450Pa
15. F = 6237N
16. E (1992 PI Q10)
17. E (1995 PI Q9)
18. B (1999 PI Q8)
19. C (1996 PI Q10)
20. m = 50kg
21. F =176,000N
22. A = 1x10-2m2 (using g=10N/kg)
23.a. A sharp blade has a small surface area.
This creates a large pressure when a force
is applied to the knife because: P = F/A.
b. Skis increase the contact area with the snow.
The pressure produced by the weight of the person
is therefore reduced (P = F/A).
24. P = 18849.56N
25. Fres = 15x104N (to the left) (1993 PI Q32)
Pressure in a Fluid
26. E (1998 PI Q8)
27. B (1992 PI Q12)
Buoyancy/Upthrust
28. A (1997 PI Q10)
29. D (1999 PI Q6)
30. E (1992 PI Q6)
31. All forces on the balloon are balanced [N1].
The weight of the balloon is equal and opposite
to the upthrust force.
32a. (1997 PI Q32a)
 b. V = 0.14cm3
c. Plot a graph of volume against kelvin
temperature for the known temperatures of
boiling water and melting ice.
Record the volume for the freezing mixture
and use the the graph to estimate the kelvin
temperature of the mixture.
Or
Use the relationship:
V1/T1 = V2/T2
And solve for the unknown temperature(T2) by substituting
in known values of V1,T1,V2 and solving for T2.
d. The relationship: V1/T1 = V2/T2, assumes the gas is
at constant pressure, equal to atmospheric pressure.
A change in atmospheric pressure would change the gas pressure.
A graph, or constant(P/T), would therefore be required for each
pressure of the gas.
Pressure and Volume
52. E (1992 PI Q11)
53. A (1995 PI Q10)
54. When a gas is compressed into a smaller volume
the particles collide more frequently with the
walls of their container. A geater average
force is therefore exerted by the gas on a
smaller area. This is equivalent to an increase
in pressure.
55. V = 1500(litres)
56. V = 56ml
57a. V = 1800(litres)
b. 900 litres available at 200kPa
time = available volume/volume per minute
t = 900/25
t = 36(minutes)
58. P = 4x105Pa
59. V2 = 3.84m3 (1995 PI Q33)
60a. (1996 PII Q4a)
b. V = 0.14cm3
c. Plot a graph of volume against kelvin
temperature for the known temperatures of
boiling water and melting ice.
Record the volume for the freezing mixture
and use the the graph to estimate the kelvin
temperature of the mixture.
Or
Use the relationship:
V1/T1 = V2/T2
And solve for the unknown temperature(T2) by substituting
in known values of V1,T1,V2 and solving for T2.
d. The relationship: V1/T1 = V2/T2, assumes the gas is
at constant pressure, equal to atmospheric pressure.
A change in atmospheric pressure would change the gas pressure.
A graph, or constant(P/T), would therefore be required for each
pressure of the gas.
Pressure and Volume
52. E (1992 PI Q11)
53. A (1995 PI Q10)
54. When a gas is compressed into a smaller volume
the particles collide more frequently with the
walls of their container. A geater average
force is therefore exerted by the gas on a
smaller area. This is equivalent to an increase
in pressure.
55. V = 1500(litres)
56. V = 56ml
57a. V = 1800(litres)
b. 900 litres available at 200kPa
time = available volume/volume per minute
t = 900/25
t = 36(minutes)
58. P = 4x105Pa
59. V2 = 3.84m3 (1995 PI Q33)
60a. (1996 PII Q4a)