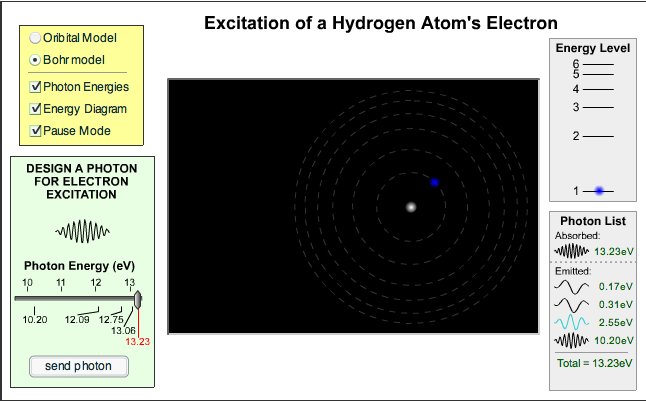
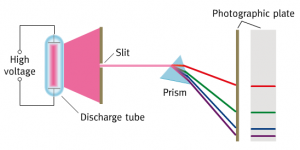
We’ve looked at line spectra with spectroscopes and a spectrometer recently. Now we’re considering how these different colours of light are produced.
Here is a website that lets you choose the energy of a photon and see whether or not it causes a change in the energy of an electron inside the hydrogen atom. Try it – the labels under the slider for photon energy match the electron transitions.
You can read more about line spectra and their origin here.
The visible line spectrum of the Hydrogen atom is explained in this short animation. Click on the image below to start the clip.
While you’re watching the clip, try to explain why the video does not include ANY of the possible transistions back down to the ground state.
I’ve attached a pdf file with further notes on line spectra and the absorption/emission of photons. Click on the link below to download your copy.